The FDA on August 15, 2011 approved of a novel dissolvable sinus implant that slowly releases mometasone furoate steroid (same active ingredient as Nasonex steroid nasal spray) directly onto the sinus mucosa.
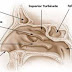
The spring-like implant is placed at the time of sinus surgery and automatically expands and conforms to the sinus cavity regardless of size and shape.
The theory is that by continuously delivering a low dose steroid directly to the sinus cavity, sinus inflammation will decrease and risk of scarring will decrease resulting in an overall decrease in chronic sinusitis for the patient after sinus surgery.
This is a GREAT idea and studies have born out its effectiveness. Hopefully other drug-eluting dissolvable stents like this will be developed in the future!
Developed by Intersect ENT, the implant is called "Propel."
Read about the FDA approval here.
References:
Safety and efficacy of a novel bioabsorbable, steroid eluting sinus stent. INTERNATIONAL FORUM OF ALLERGY & RHINOLOGY. 2011;1(1):23-32.
Advances in the Surgical Management of Chronic Sinusitis and Nasal Polyps. CURRENT ALLERGY AND ASTHMA REPORTS. Published online 08 February 2011
Controlled steroid delivery via bioabsorbable stent: Safety and performance in a rabbit model. AMERICAL JOURNAL OF RHINOLOGY & ALLERGY. November–December 2009, Vol. 23, No. 6.
Tuesday, 30 August 2011
Nasonex Sinus Implant for Chronic Sinusitis
Posted on 07:38 by Unknown
Posted in dissolvable, endoscopic, ess, implant, nasonex, propel, sinus surgery, sinusitis, surgery
|
No comments
Subscribe to:
Post Comments (Atom)















0 comments:
Post a Comment